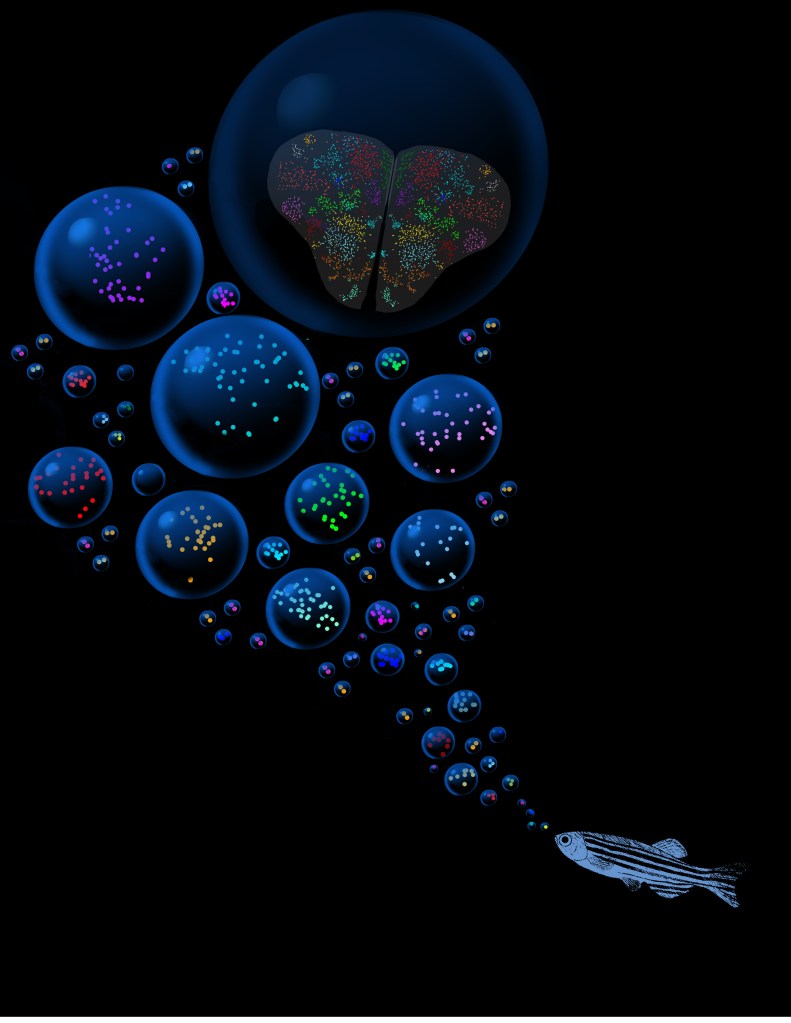
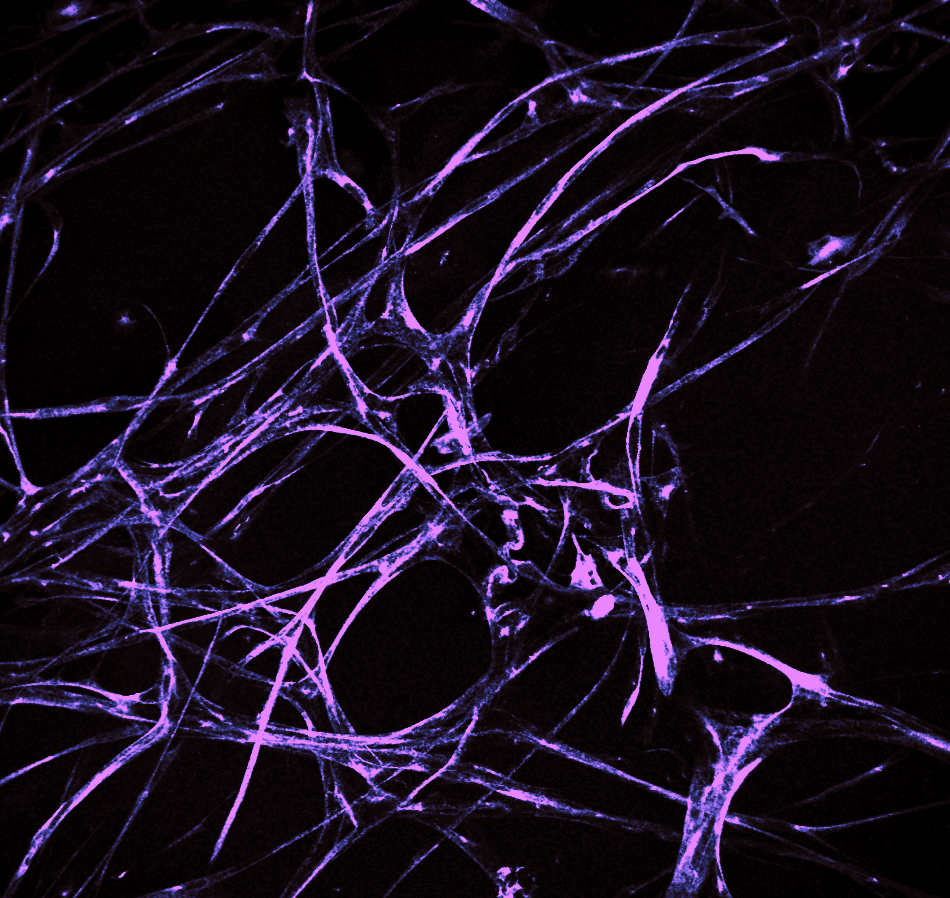
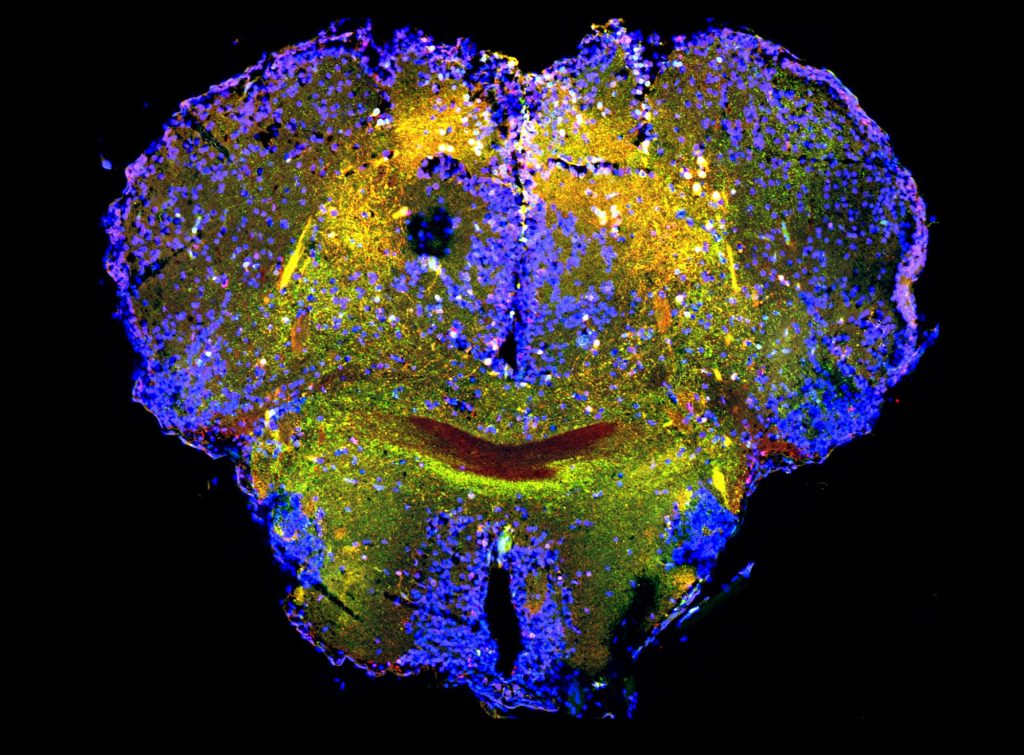
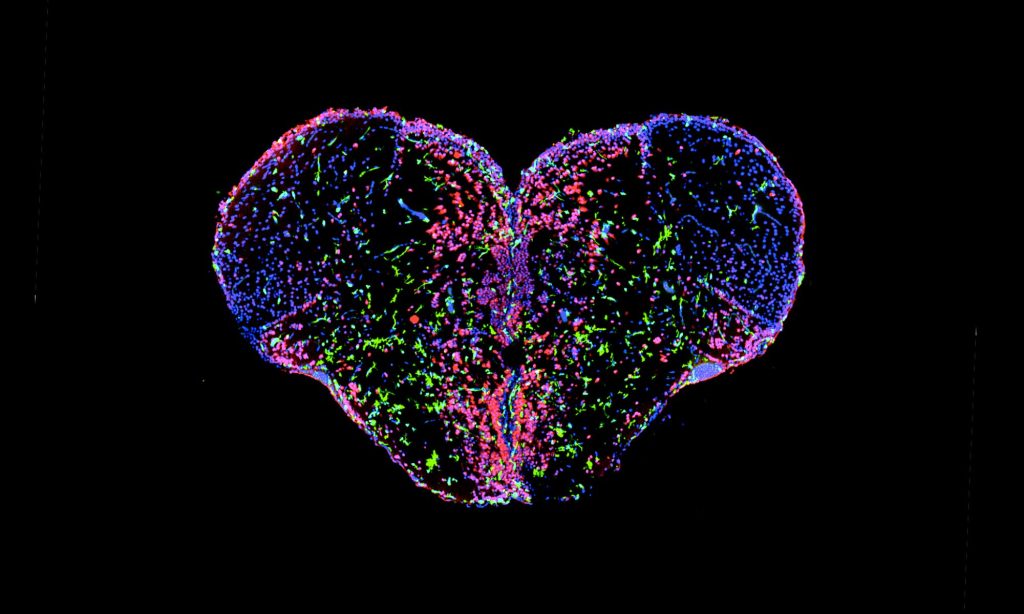
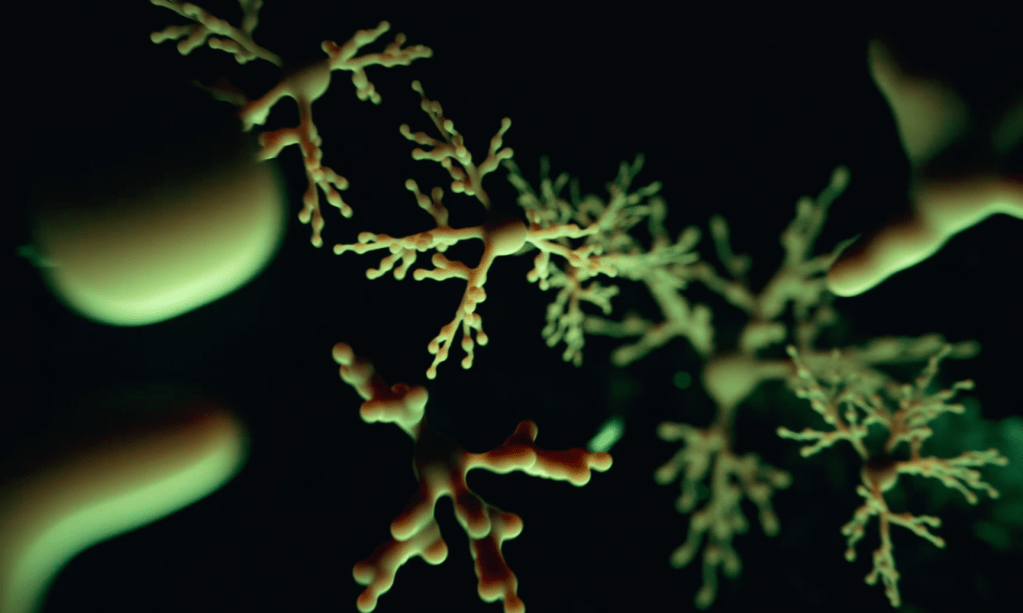
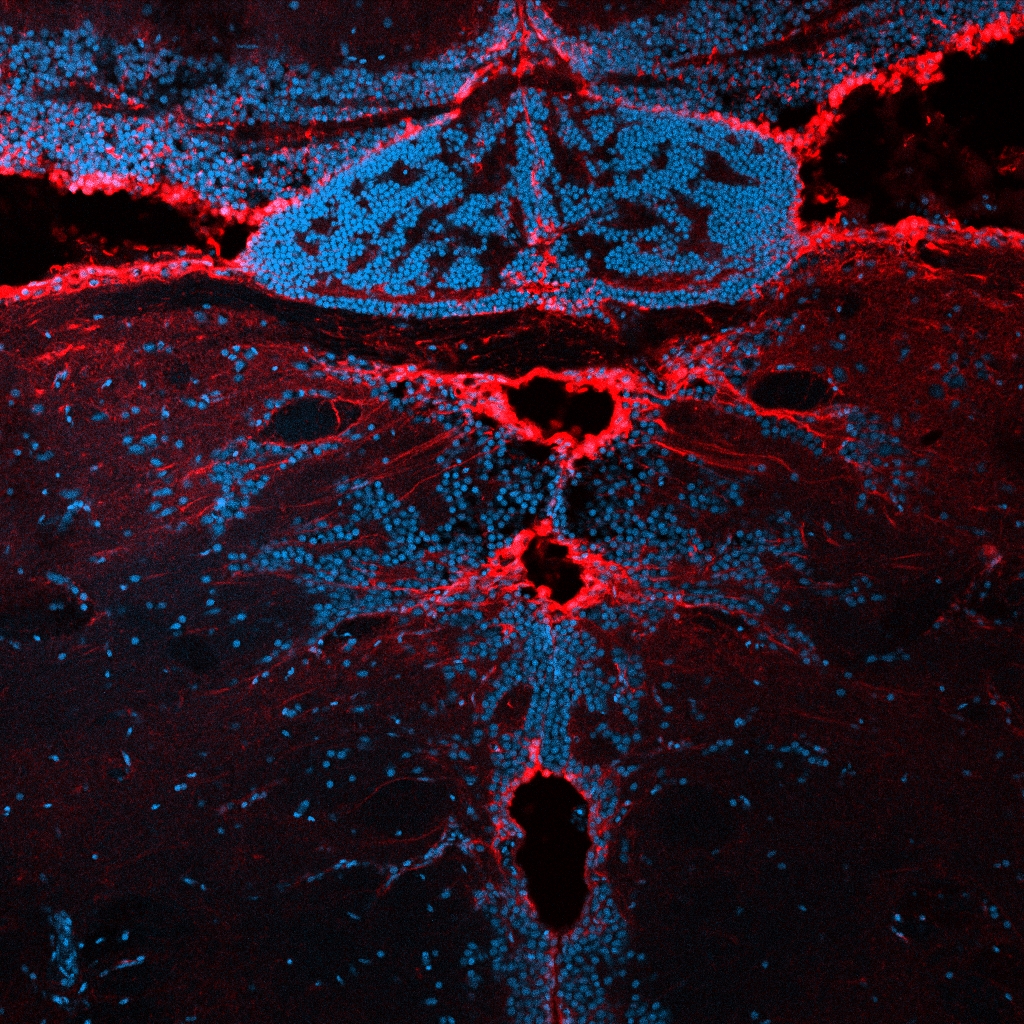
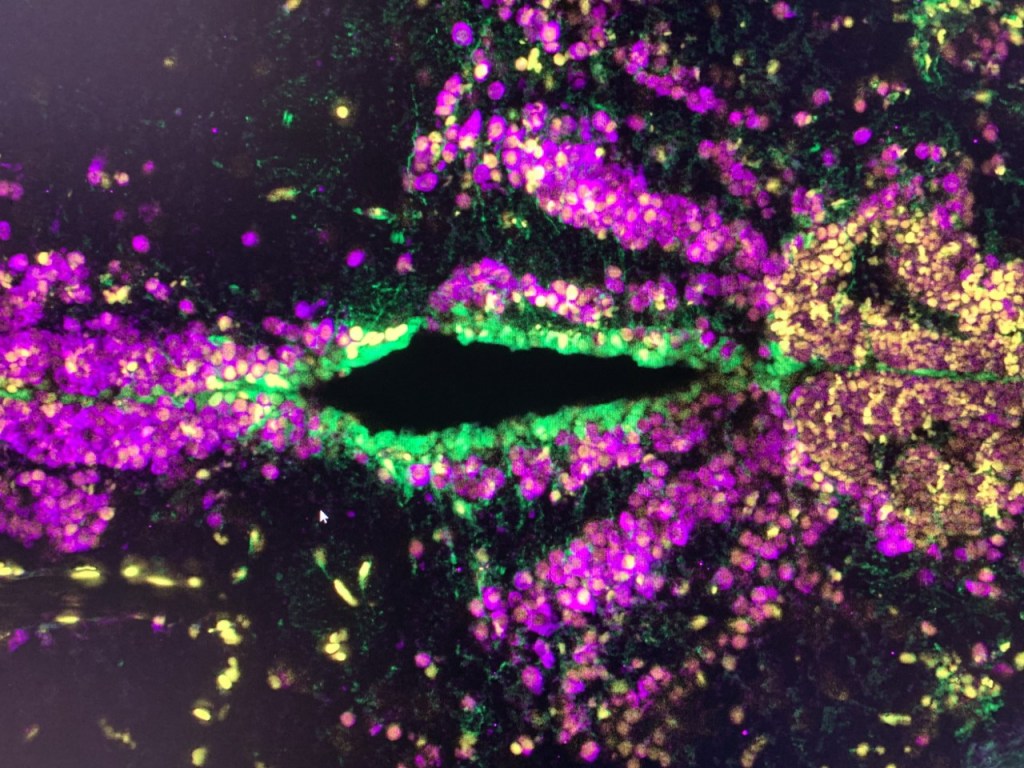
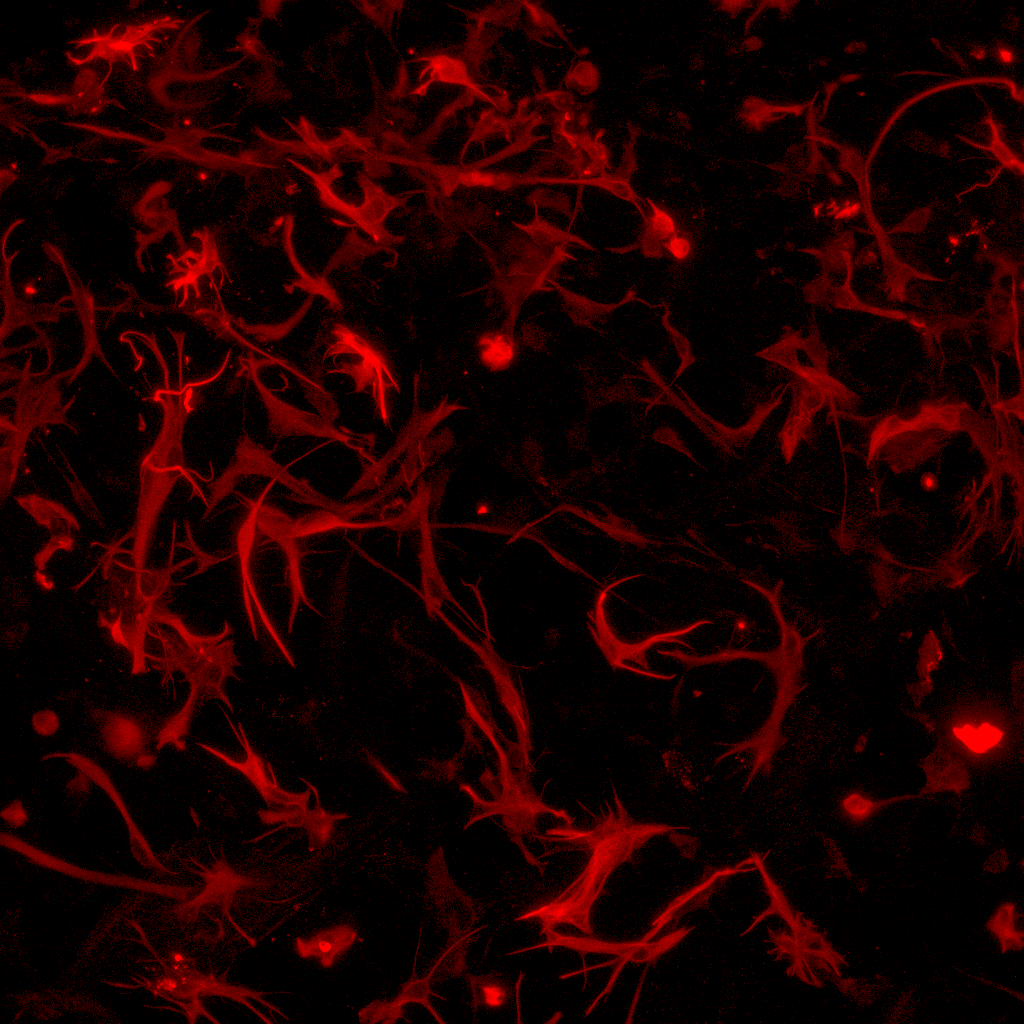
In our laboratory, we investigate the pathological mechanisms of Alzheimer’s disease in the zebrafish and mouse as well as three-dimensional human neuronal cultures from a molecular and functional genomics perspective.
Our Model Systems and Translational Pipeline
We combine zebrafish and mouse models, 3D in vitro brain models , and human tissue and large omic datasets to study molecular and cellular mechanisms of neurodegeneration, and how these pathologies can be counteracted. Zebrafish provide rapid, large-scale insight into regenerative programs and drug discovery. We validate our siscoveries in human-derived cell cultures, 3D models and mouse models, ensuring translational relevance. Together, these systems allow us to identify targets that can move from discovery to therapy.
We use zebrafish as a model to investigate the molecular and cellular functions of the clinically identified genes related to Alzheimer’s disease pathology in humans, translate the protective modalities of the zebrafish brain to in vivo and in vitro mammalian AD models, and perform chemical screens in pre-clinical zebrafish AD models we generated. With the help of our toolkit for multi-omics approaches, gene editing, in vitro bio-instructive cell culture models, histological approaches, behavioral measurement, electrophysiological modalities, and clinical data-driven model development, our long-term perspective is to determine novel cellular mechanisms of AD pathogenesis for identifying new biological targets for disease-modifying interventions and drug development.
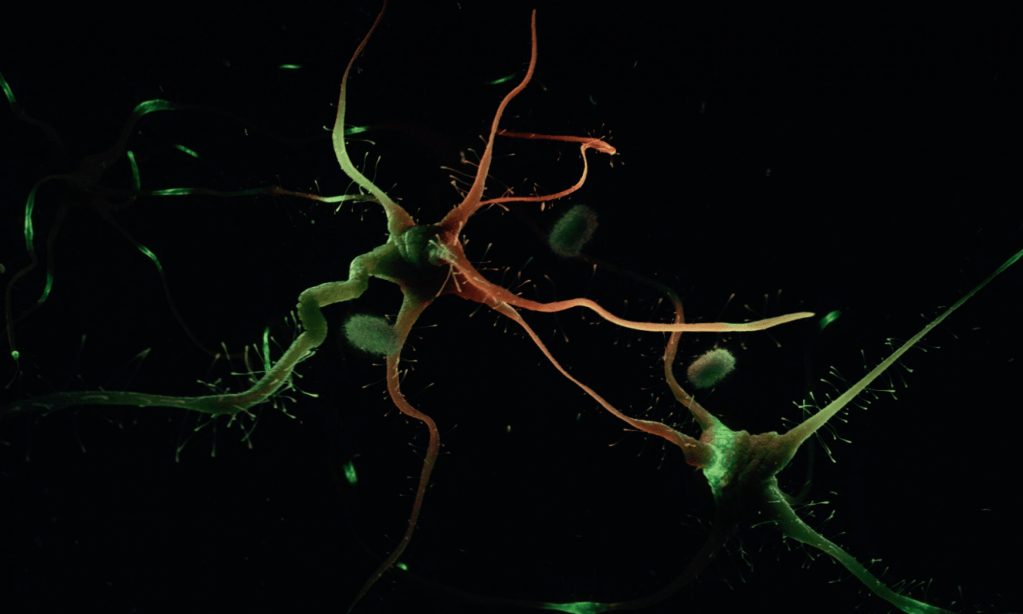
Zebrafish can also efficiently regenerate lost neurons. After generating neurodegeneration models in adult zebrafish brain, we found that the regeneration capacity of zebrafish brain depends on the induction of specific regeneration-associated programs that turn on the re-development programs of neurons. This understanding is important as to show that re-development of lost neural tissues require neural stem cells to induce special programs to mount a successful regenerative response. This is particularly important because, in a neurodegenerative state of the human brain, besides the degenerating neurons, the inability of the neural stem cells to produce new neurons is a barrier to remedy. Additionally, even if the pathological culprits of neuronal degeneration were circumvented, the functional recovery does not take place due to the lack of new neurogenesis in mammalian nervous system. By employing endogenous neural stem cells or neural progenitors, tissue loss could be counteracted, and the integrity of the existing tissues could be enhanced. Thus, neurogenesis relates to the resilience to neurodegeneration. However, the mechanism of how neuro-regeneration could be induced on demand, how AD pathogenesis alters the neurogenic cell populations, and the complex cellular crosstalk for resilience mechanisms are unknown. Neurodegenerative diseases can also be considered as “stem cell diseases”. Our research involves understanding the regenerative and developmental programs of neural stem cells in zebrafish to induce a successful proliferation-differentiation-development cascade for neurons in human brains, and harnessing this knowledge for designing stem cell-based therapies to cure Alzheimer’s disease in humans.
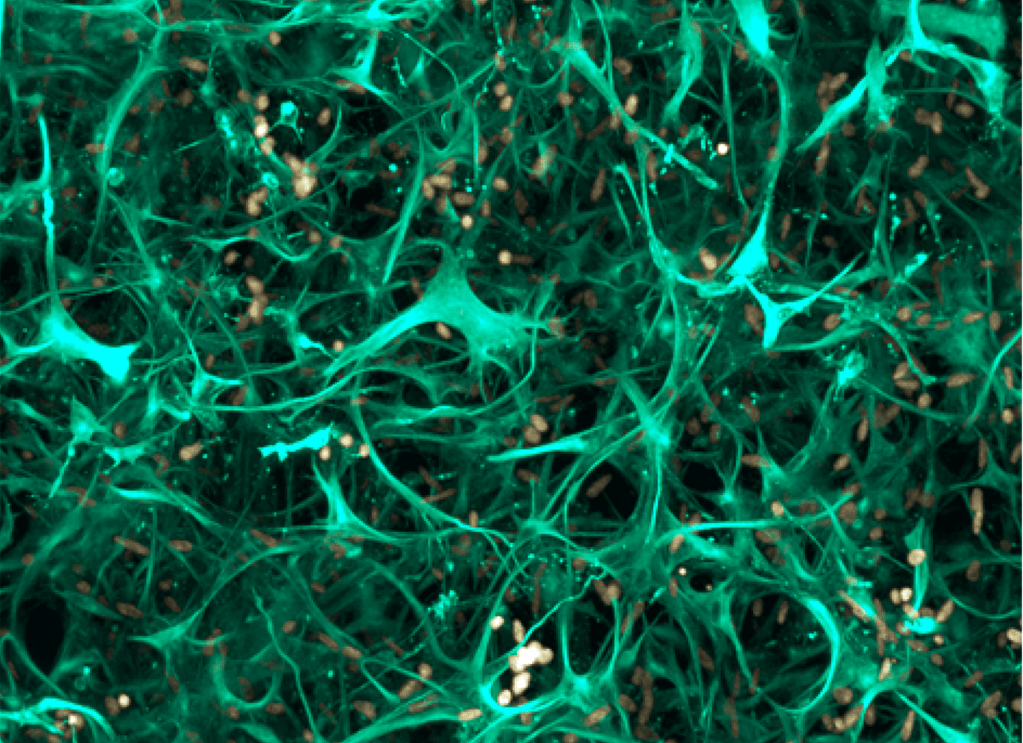
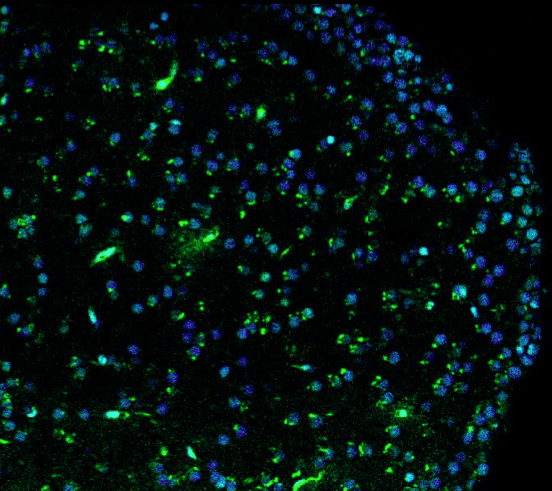
To serve as a comparative experimental model for zebrafish, we have also generated in vitro 3D human brain culture system by using neural stem cells that lead to tissue-mimetic modeling of human neuronal development and degeneration. By using these two systems in a comparative manner, we have identified several regeneration factors that facilitate human neural development and ameliorate hampered plasticity of human neural stem cells in disease conditions.
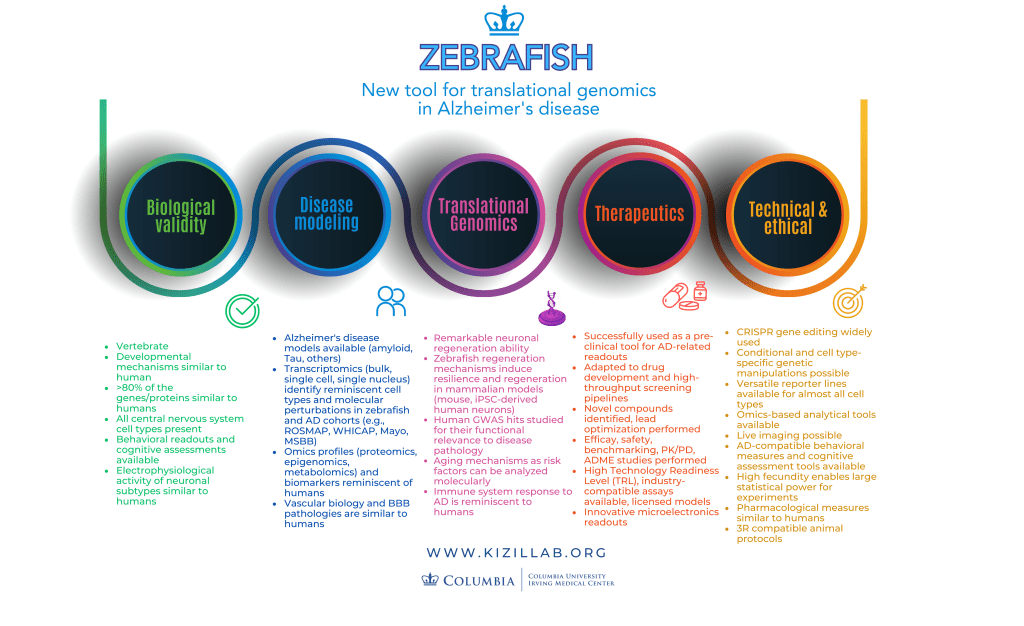
What We Deliver
- Validated orthologous zebrafish models for studying neurodegenerative mechanisms and vascular pathology.
- Drug screening pipelines identifying compounds for modifying neuronal survival, synaptic strength, neuroinflammation, vascular health, neuroregeneration and other key pathologies in Alzheimer’s disease.
- Quantitative behavioral and imaging assays for high-throughput testing.
- Open-access data and protocols to accelerate discovery across labs.
- A commitment to training and inclusion, preparing the next generation of neuroscientists.